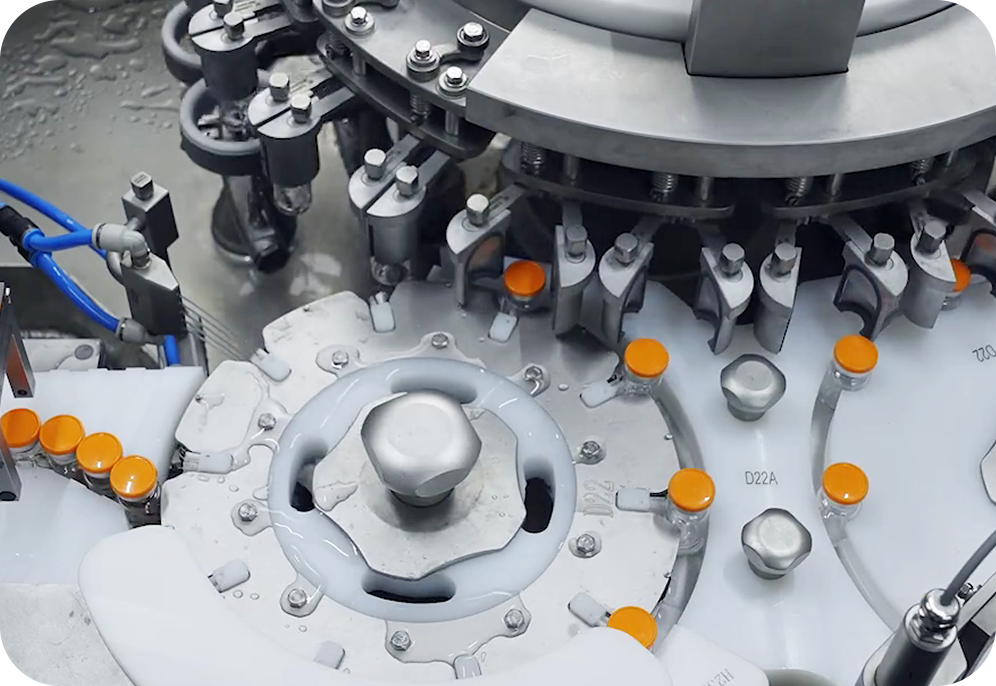
Start Bioservices is a trusted CDMO partner with the expertise and capacity to support in the process development, manufacturing and commercialization of mRNA therapies. Our services range from plasmid and in vitro transcription (IVT) to lipid nanoparticle (LNP) encapsulation and fill-finish, ensuring high quality, cost-effective and timely production.
Why Choose Us
mRNA Manufacturing Process
mRNA Analysis Methods and Specifications
mRNA Analysis
Methods and
Specifications
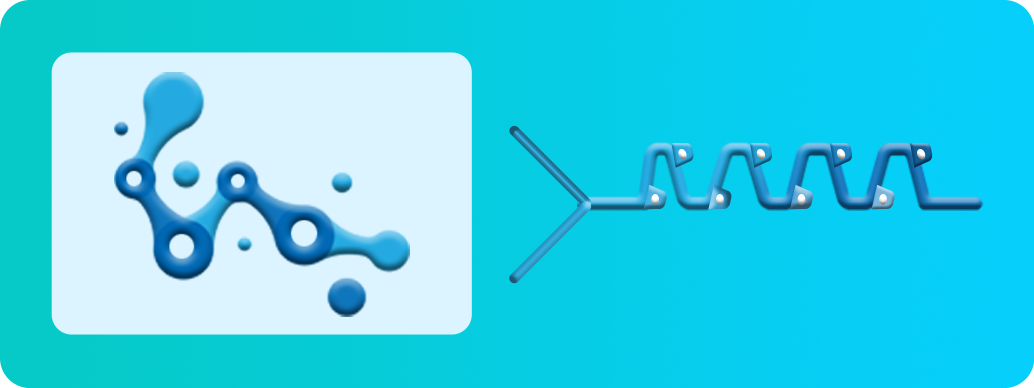
mRNA-LNP Encapsulation Process
Aqueous
Phase mRNA
Ethanolic Phase
Lipids
Microfluidic
Mixing
mRNA-LNPs
Formulation
Dilution
Formulation Buffer
mRNA-LNP Ultrafiltration
& Diafiltration
mRNA-LNPs
Drug Product